O. Klepitskaya, R. Kumar, University of Colorado Health Sciences Center, Department of Neurology, Aurora, CO, USA; Colorado Neurological Institute Movement Disorder Center, Englewood, CO, USA.
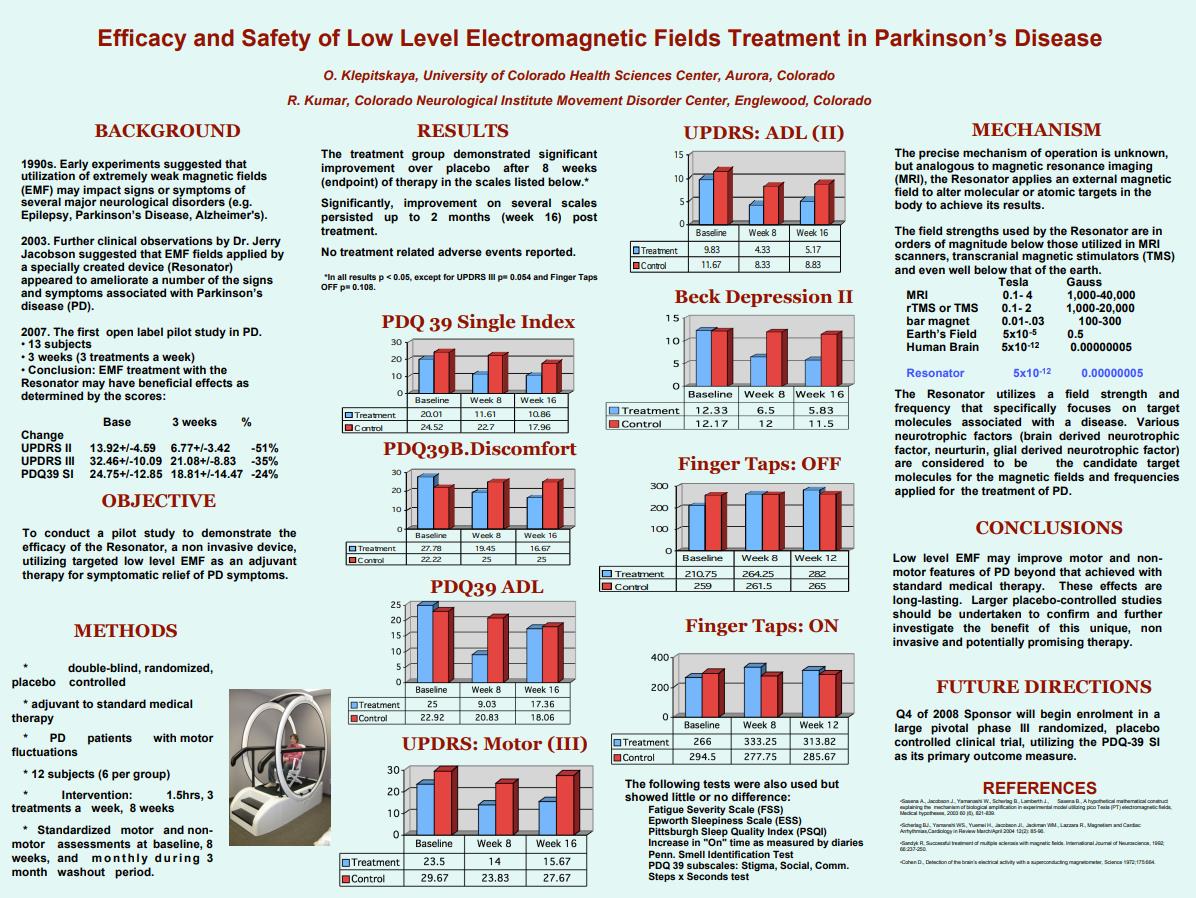
Background:
Small case series suggest extremely low level (10212Tesla) electromagnetic fields (EMF) may be useful in the treatment of Parkinson’s disease (PD). No controlled studies have been previously reported.
Design/Methods:
A single center, double blind, randomized, placebo controlled trial of EMF as an adjuvant to standard medical therapy in PD patients with motor fluctuations was performed in 12 subjects (6 per group). 24 sessions of 1.5 hour of total body EMF were administered over 8 weeks. Standardized motor and non-motor assessments were performed prior to treatment, at endpoint, and monthly for 3 months.
Results:
The treatment group demonstrated significant improvement over placebo after 8 weeks of therapy as follows: Scale, absolute point reduction, % improvement vs % improvement placebo (unless noted all results p.05): UPDRS II(ON) 5.5, 56% vs 28%; UPDRS III(ON) 9.5, 40% vs 20%, p5.054; PDQ-39(SI) 8.4, 42% vs 7%; PDQ-39(MOB) 11.67, 47% vs 6%; PDQ-39(ADL) 15.97 pts, 64% vs 9%; PDQ-39(BD) 8.33 pts, 30% vs -13%; Beck Depression Inventory II 5.73 pts, 47% vs 1%; Fatigue Severity Scale 7.66 pts, 22% vs 5%, p5.12; Finger Taps (ON) 67 taps, 25% vs -5%. Importantly, improvement on several scales persisted up to 2 months post treatment. No treatment related adverse events reported.
Conclusions:
Low level EMF may improve motor and non-motor features of PD beyond that achieved with standard medical therapy. These effects are long-lasting. Larger placebo-controlled studies should be undertaken to confirm and further investigate the benefit of this unique, noninvasive and potentially promising therapy.
Research published in the Heart Rhythm Society journal Heart Rhythm O2 concluded: "In this first-in-human, proof-of-concept study in patients with paroxysmal AF, LL-EMF stimulation results in significantly shorter episodes of pacing-induced AF, as well as a reduced likelihood of spontaneous firing initiating an episode of AF, compared to sham stimulation. Larger studies are warranted to confirm these findings and provide further mechanistic insights."
Key Findings

J.I. Jacobson 1, R. Gorman 1, F. Chaviano 1, W.S. Yamanashi 2, I. Grinberg 3, M. Dayton 4, S. Haltiwanger 5, B. B. Saxena 6, B. Walters 7, L. Clayton 8, J. Lamberth 9
Dr. Pedro Alonso Atienza; Costa del Sol Hospital, Marbella, Spain
Dr. Joaquin Garcia Montes; Serrania Ronda Hospital, Spain
O. Klepitskaya 1, R. Kumar 2 (2009)
1 University of Colorado Health Sciences Center, Department of Neurology, Aurora, CO, USA
2 Colorado Neurological Institute Movement Disorder Center, Englewood, CO, USA.
By Jerry Jacobson, PhD, DMD, IOM
Published in Dynamic Chiropractic – July 15, 2013, Vol. 31, Issue 14
Daniel Sohinki, MD, MS 1, Stavros Stavrakis, MD, PhD 2, Sunny Po, MD, PhD 2, Julie A. Stoner, PhD 2, Benjamin J. Scherlag, PhD 2 1 Medical College of Georgia, Augusta University, Augusta, Georgia 2 University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma (Nov 2019)
Songyn Wang 1, Xiaoya Zhou 1, Zhuo Wang 1, Bing Huang 1, Liping Zhou 1, Mingxian Chen 2, Lilei Yu 1, Hing Jiang 1 1 Department of Cardiology, Renmin Hospital of Wuhan University, Cardiovascular Research Institute of Wuhan University, Wuhan, Hubei, China; 2 Department of Cardiology, The Second Xiangya Hospital of Central South University, Changsha 410011, Hunan, China
International Journal of Cardiology 190 (2015) 54-55
Jerry I Jacobson, Institute of Theoretical Physics and Advanced Studies for Biophysical Research, Jupiter, FL, USA (2016)
Jerry I. Jacobson, Institute of Theoretical Physics and Advanced Studies for Biophysical Research, Jupiter, FL, USA (2016)
Jacobson J I, Institute of Theoretical Physics and Advanced Studies for Biophysical Research, Jupiter, Florida, USA Published in Innovation Energy & Research 2016, 5:1
Lilei Yu, MD 1, John W. Dyer, PhD 2, Benjamin J. Scherlag, PhD 2, Stavros Stavrakis, MD, PhD 2, Yong Sha, MD 2, Xia Sheng, MD 2, Paul Garabelli, MD 2, Jerry Jacobson, DMD, PhD 3, Sunny S. Po, MD, PhD 2 1 Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, China; 2 Heart Rhythm Institute and Department of Medicine, Oklahoma Health Sciences Center, Oklahoma City, Oklahoma; 3 Pico-Telsa Therapies LLC, Littleton, Colorado (2015)
Jerry Jacobson 1, Benjamin Sherlag 2, 1. Institute of Theoretical Physics and Advanced Studies for Biophysical Research, Jupiter, Florida, USA; 2. Department of Medicine, Cardiovascular Section, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA (2015)
By Jerry Jacobson, DMD, PhD, IOM Published in The American Chiropractor September 2015
Jerry Jacobson 1, Benjamin Sherlag 2
1 Institute of Theoretical Physics and Advanced Studies for Biophysical Research, Jupiter, FL, USA 2; Department of Medicine, Cardiovascular Section, University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA (2015)
Jacobson J I, Institute of Theoretical Physics and Advanced Studies for Biophysical Research, Jupiter, Florida, USA International Journal of Clinical & Medical Imaging, August, 2015
Chao Qin, MD, PhD 1, J. Mark Evans, MD 1, William S. Yamanashi 2, PhD, Benjamin J. Scherlag, MD 2, Robert D. Foreman, PhD 1 Departments of 1 Physiology and 2 Medicine, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma
© 2005 International Neuromodulation Society
Benjamin J. Scherlag 1, PhD, William S. Yamanashi 1, PhD, Yuemei Hou MD 2, PhD, Jerry I. Jacobson 3, PhD, Warren M. Jackman 1, MD, Ralph Lazzara, MD 1
1. Cardiac Arrhythmia Research Institute at the University of Oklahoma Medical Center, Oklahoma, USA
2. Xingjiang Medical University, Urumuqui, PR, China
3. Jacobson Resonance Enterprises, Boynton Beach, Florida
C.Todd Trostel, DVM; Ron M. McLaughlin, DVM, DVSc; John G. Lamberth, PhD; Robert C. Cooper, DVM, MS; Steven H. Elder, PhD; Roy R. Pool, DVM, PhD; Cheng Gao, DDS, MS; Joseph A. Cromiak, PhD; Carolyn R. Boyle, PhD
From the Departments of Clinical Sciences (Trostel, McLaughlin, Cooper), Pathobiology and Population Medicine (Pool), and Basic Sciences (Gao, Boyle), College of Veterinary Medicine; the Department of Health, Physical Education, Recreation, and Sport (Lamberth, Cromiak), College of Education; and the Department of Agricultural and Biological Engineering (Elder), College of Agriculture and Life Sciences, Mississippi State University, Mississippi State, MS.
American Journal of Veterinary Research, Vol 64, No. 7, July 2003
Anjali Saxena,1 Jerry Jacobson,2 William Yamanashi,2 Benjamin Scherlag,3 John Lamberth,4 Brij Saxena 5
1. Department of Biological Sciences, Fairleigh Dickinson University, Teaneck, New Jersey, USA.
2. Institute of Theoretical Physics and Advanced Studies for Biophysical Research, Perspectivism Foundation, 2006 Mainsail Circle, Jupiter, Florida 33477, USA.
3. Department of Medicine, Cardiovascular Section, University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, USA.
4. Department of Recreation, Sports and Physical Therapy, Mississippi State University, Mississippi, USA.
5 Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology, Weill Medical College of Cornell University, NY, USA.
Five Institutional Review Boards (IRBs), including Independent Review Consulting, Inc. IRB, Western Investigational Review Board (WIRB), University of Colorado’s IRB (COMIRB), Ethical & Independent Review Services, and University of South Florida’s IRB have determined the predecessor to the PEMF Device, the Jacobson Resonator™, to be a non-significant risk (NSR) device when applied to the particular studies and protocols identified below. These protocols involved Parkinson Disease, Alzheimer Disease, Fibromyalgia, and Type 2 Diabetes patients for eight independent studies, as described below:
Efficacy of the application of magnetic fields to the treatment of Parkinson’s disease pilot study protocol, APPLIED MAGNETICS, LLC. Version 1.6, February 20, 2007. IRC study # 07021-02.
A double-blind, sham-stimulation controlled study of the application of magnetic fields using the Jacobson Resonator™ for the treatment of Parkinson’s disease: phase two pilot study protocol. Version 2.5, Oct 2007. IRC # 07102-01.
An open label extension study for subjects previously treated in either pilot I or pilot II studies of the application of magnetic fields using the Resonator™ for the treatment of Parkinson’s disease: Extension pilot study protocol, Version 4.0, March 14, 2008. IRC# 06052-01.IRC Resonator Device NSR #: DAM-003.
A controlled pilot study of the application of magnetic fields using the Resonator™ in adjunctive management of Type 2 Diabetes; Version 1.8, December 2, 2008; Pico-Tesla Magnetic Therapies, LLC., a subsidiary of APPLIED MAGNETICS, LLC. IRC # 08165-01
A randomized, double-blind, placebo-controlled study of the application of magnetic fields using the Resonator™ in adjunctive management of Type 2 Diabetes Mellitus, pilot study II. IRC # 09109-01
A multiple site, randomized, double-blind, sham-stimulation controlled study of the application of magnetic fields using the Resonator™ device for the treatment of Parkinson’s disease; Pico-Tesla Magnetic Therapies, LLC. April 7, 2010. IRC # 09026:
A single site, randomized, single-blind, placebo-controlled study to evaluate the safety and efficacy of the application of magnetic fields using the Resonator for the treatment of Alzheimer’s Disease in addition to standard of care, Pico-Tesla Magnetic Therapies, LLC. October 12, 2010. USF IRB Study # Pro00001676
A randomized, double-blind, placebo-controlled, evaluation of the application of magnetic fields using the Resonator™ device for the treatment of fibromyalgia: pilot study protocol. Pico-Tesla Magnetic Therapies, LLC. November 2, 2010, E&I Review Services Study # 10242-01
All research and clinical material is for informational purposes only. Readers are encouraged to confirm the information contained herein with other sources. The information is not intended to replace medical advice offered by physicians.